As of December 9, 2021, the FDA authorized 422 tests and sample collection devices under emergency use authorization (EUAs). Of those, at least 67 are COVID home collection samples. StatsFind compiled COVID home collection statistics from these approved tests to discover which are most effective.
Understanding the statistical components of what makes one test different from another can help you evaluate the proper kit for your circumstances. Most international travel now requires the testing discussed in this article.
Let’s explore the statistical differences between the COVID home collection tests available in the marketplace.
Highlights:
- There is a difference between at-home COVID kit testing and home collection testing.
- COVID home collection is an easy choice for those who want a high-quality test from home.
- COVID home collection tests have a high statistical efficacy in detecting or excluding COVID-19
- The majority of COVID home collection kits detect the new Omicron variant.
COVID self-test kits – What’s the difference between at-home and home collection testing?
Self COVID-19 home kit testing involves collecting and processing the tests yourself. The majority of these tests are called antigen tests. More details are available in the previous post:
Here, we are going to focus on home collection testing.
Home collection testing involves collecting the test yourself and then sending the test to a clinical laboratory for processing. This type of testing is typically called a polymerase chain reaction (PCR) test. PCR stands for a kind of testing that amplifies the genetic material of COVID-19 to detect the smallest amount of coronavirus. PCR testing is the “gold standard” in diagnosing COVID-19, and the results are usually ready in two to three days.
The following video contains more information on COVID-19 tests:
How to do a home collection test
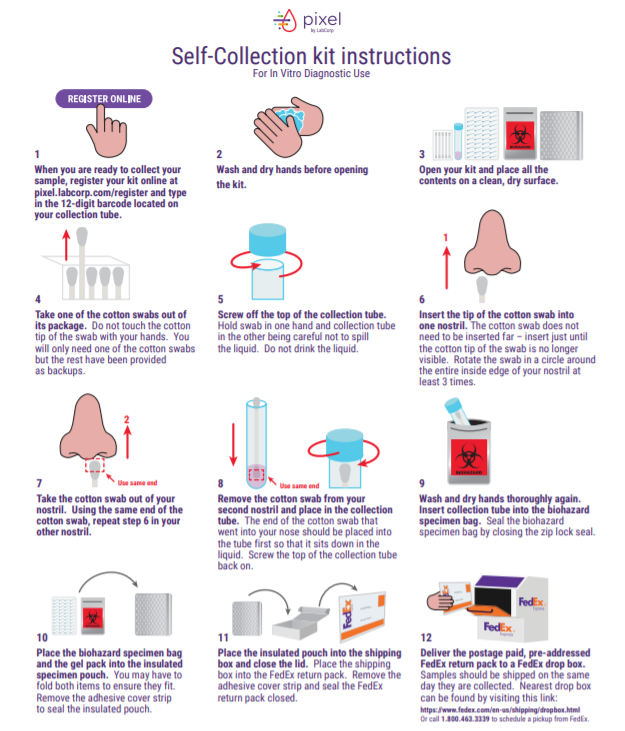
COVID home collection is an easy choice for those who want a high-quality test from home and are looking to avoid going out for testing. The subsequent image is an example from Pixel Labcorp, and individuals apply the same general procedures when performing a home collection test.
Where do I find the latest COVID home collection tests approved in the United States?
The FDA authorizes COVID-19 home collections testing. As of the date of this article, 100% of the home collection tests have emergency use authorization for usage in the United States. EUA signifies that the FDA has authorized an unapproved medical product for use in the diagnosis treatment or prevention of a severe or life-threatening illness, in this case, COVID-19. EUAs indicate sufficient evidence exists that the home collection test will be beneficial.
For a complete list of approved tests, please visit the FDA website.
There are hundreds of approved tests on the FDA’s website. However, you must enter “home collection” in the search field to narrow it down to those considered in this article.
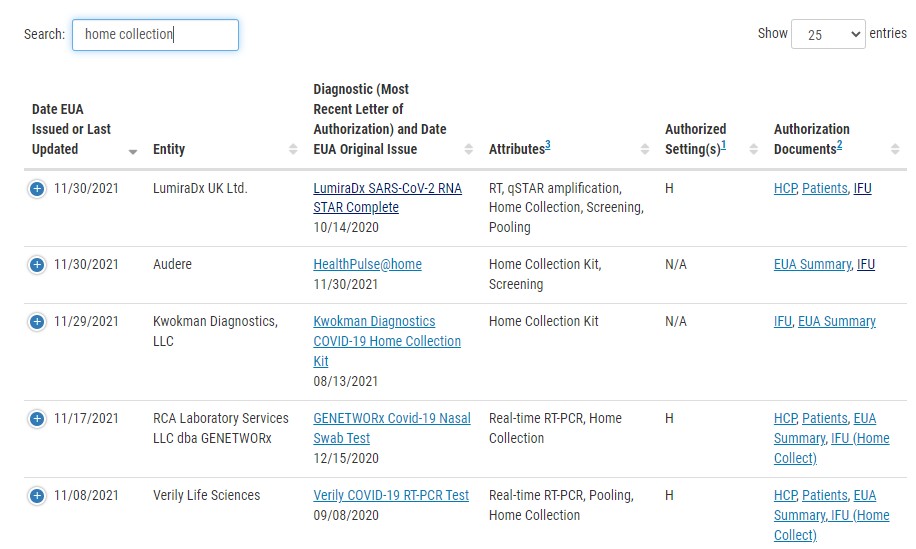
Many companies possess approved tests but have not made them available to the general public. We focused on the most readily available PCR testing that the consumer can purchase today.
Terms used in COVID home collection statistics
Covid home kit statistics use key terms for measuring the accuracy of the testing.
- Sensitivity: detects a true positive test indicating COVID-19. A similar term is positive percent agreement (PPA) when using a non-reference standard.
- Specificity: detects a true negative test indicating the person does not have active disease. A similar term is negative percent agreement (NPA) when using a non-reference standard.
- Symptomatic: The patient is showing symptoms of COVID-19 as defined by the CDC.
- Asymptomatic: The patient has no symptoms
- Omicron: The new (B.1.1.529) variant spreading across the globe
COVID home collection statistics
In the following chart, we summarized a collection of statistics of the most common commercially available COVID home collection tests. They range from the highest PPA to the lowest and are all PCR. The last column displays if a manufacturer included a statement on whether their product detected the new Omicron variant as of December 12, 2021.
| COVID AT-HOME TEST KIT | SYMPTOMATIC SENSITIVITY/PPA | SYMPTOMATIC SPECIFICITY /NPA | ASYMPTOMATIC SENSITIVITY/PPA | ASYMPTOMATIC SPECIFICITY /NPA | OMICRON |
| Pixel by Labcorp COVID-19 Test Home Collection Kit | N/A | N/A | 100.00% | 100.00% | Yes |
| VAULT (Infinity BiologiX TaqPath SARS-CoV-2 Assay) | N/A | N/A | 100.00% | 100.00% | Yes |
| VITAGENE (Infinity BiologiX TaqPath SARS-CoV-2 Assay) | N/A | N/A | 100.00% | 100.00% | Yes |
| Cue COVID-19 Test for Home and Over The Counter (OTC) Use | 96.40% | 98.20% | 100.00% | 100.00% | Yes |
| LetsGetChecked | N/A | N/A | 100.00% | 99.20% | Yes |
| empowerDX at-Home COVID-19 Nasal PCR Test, FDA Authorized (Home-Collected)* | N/A | N/A | 100.00% | 99.10% | Yes |
| Picture (Fulgent Genetics) | 100.00% | 100.00% | 100.00% | 98.30% | Yes |
| Amazon COVID-19 Test Collection Kit DTC (STS Lab Holdco)* | N/A | N/A | 99.00% | 99.40% | Unknown |
| Phosphorus RT-qPCR test | N/A | N/A | 97.10% | 98.20% | Unknown |
| Everlywell COVID-19 Test Home Collection Kit DTC | N/A | N/A | 95.00% | 98.00% | Unknown |
| DxTerity COVID-19 Saliva at-Home Collection Kit* | 97.30% | 90.00% | 84.60% | 99.00% | Yes |
How will you use the COVID home collection statistics?
As the chart shows, COVID home collection tests have a high statistical efficacy in detecting or excluding COVID-19. The majority of COVID home collection kits detect the new Omicron variant.
We can assume that the tests that don’t contain a company comment or press release on detecting Omicron will have one soon since most PCR testing can detect the Omicron variant.
Statistics are essential to determine the best test for you to use. However, active symptoms of COVID-19 should be tested immediately and acquiring whatever testing is the most available to you is also of utmost importance.
Watch our site for reports on updated and approved COVID-19 home collection tests available on the market and their statistics.
The information contained in this blog is for educational and informational purposes only and is not intended as health or medical advice. Always consult a physician or other qualified health provider regarding any questions about a medical condition or health objectives.
Additional references:
- https://www.whitehouse.gov/briefing-room/speeches-remarks/2021/12/02/remarks-by-president-biden-on-the-covid-19-winter-plan/
- LumiraDx SARS-CoV-2 RNA STAR Complete is a rapid nucleic acid amplification technology removing the need to perform extraction and purification steps.
- DxTerity SARS-CoV-2 RT PCR CE Test – EUA Summary (fda.gov)
- Amazon Multi-Target DTC Test – EUA Summary (fda.gov)
- Clinical Enterprise SARS-CoV-2 RT-PCR Assay DTC – EUA Summary (fda.gov)
- Viracor Eurofins Clinical Diagnostics Viracor SARS-CoV-2 Assay – EUA Summary (fda.gov)
- Pixel by Labcorp COVID-19 Test Home Collection Kit – EUA Summary (fda.gov)
- COVID-19 RT-PCR Test – EUA Summary (fda.gov)
- LabCorp COVID-19 RT-PCR test EUA Summary
- At-Home COVID-19 (Coronavirus) Test – COVID-19 Test Home Collection Kit DTC | Everlywell
- LetsGetChecked Coronavirus (COVID-19) Test – EUA Summary (fda.gov)
- Omicron Variant: 6 Things to Know About the Latest COVID-19 Variant (letsgetchecked.com)
- Corporate COVID-19 Testing Programs – Vault (vaulthealth.com)
- Infinity BiologiX TaqPath SARS-CoV-2 Assay – EUA Summary .pdf
- Infinity BiologiX Help Identify First Case of Omicron in Minnesota, the Second Case Identified in the United States | Business Wire
- COVID-19 Home Saliva Test Kit: FDA EUA Authorized – Vitagene
- Phosphorus COVID-19 RT-qPCR Test DTC – EUA Summary (fda.gov)
- Fulgent COVID-19 by RT-PCR Test – EUA Summary (fda.gov)
- Fulgent Genetics Confirms Ability to Detect Omicron Variant with RT-PCR Tests for COVID-19 – Bloomberg
- Cue_COVID-19_OTC_Test_Instructions_For_Use_(IFU) (cuehealth.com)
- Cue Health Confirms COVID-19 Tests Can Detect Omicron Variant (prnewswire.com)
- Coronavirus (COVID-19) Update: December 10, 2021 | FDA




Leave a Reply